I was listening to a podcast not too long ago discussing hydration (See Huberman Lab on my podcast list (Podcasts)). The episode titled “Water and Your Health” was a typical multi-hour deep dive into all things water and hydration. I was less interested in the pH, alkaline water, and structured water.
What did stick for me was the concept of hydration in terms of solvent-solute concentrations. I recall a discussion (this podcast or a different one) on how drinking too much water on hot days or after working out could actually be detrimental. The body wants to keep a homeostasis bewteen the water in the body and the various cations such as Na+, K+, and Mg2+ just to name a few. If the concentration of these cations is too high relative to the water, drinking regular water is what your body needs in terms of rehydration. However, if your body has used or secreted salt after exercising and sweating, drinking more regular water is not ideal. In this situation, your body will work to eject that water as not to dilute the salts remaining in your system.
There are people who have died from drinking too much water in a short time. A radio contest had a contestant drank 1.5 gallons of water. She drank too much water causing the cells and the brain to expand. Drinking too much water without salts is also known to cause brain fog, cramping, and fatigue.
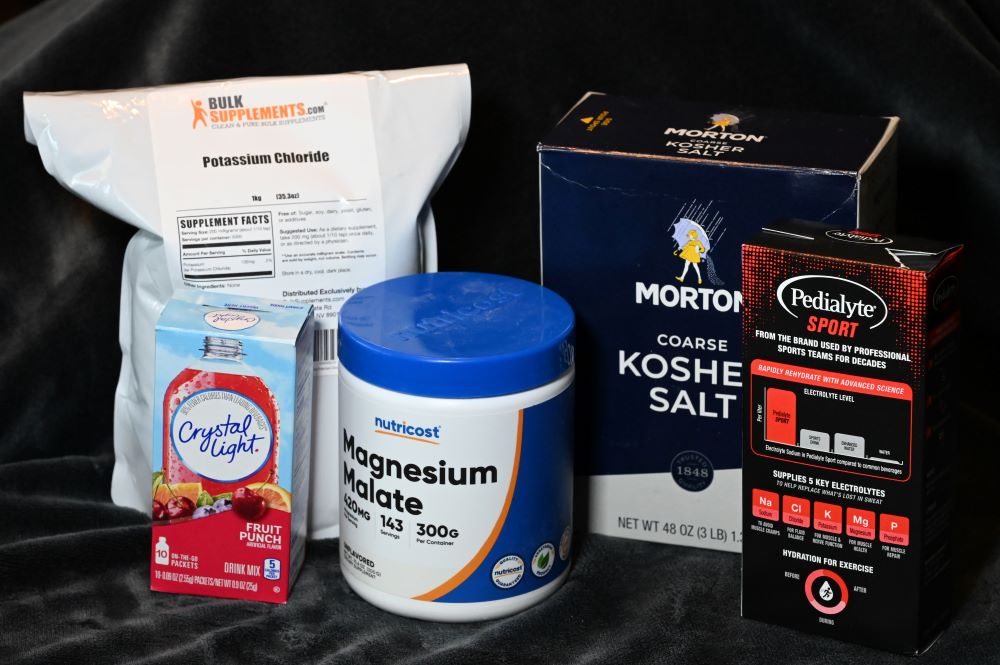
Enter the popular electrolyte drinks. The latest electrolyte drinks rail against the sugary electrolyte drinks of the past. These new drinks lower the sugar, and up the salts. One such drink “LMNT”, caters to endurance athletes by attempting to mimic the salt concentration of your sweat as to perfectly replace the water and salts you are losing while exercising. I tried one of these drinks recently called “Pedialyte Sport”. It had a nice unique salty sweet taste that I was not accustom to in a beverage. This got me thinking; I wonder if I could just make post-workout electrolyte drinks myself. Online searching of some common electrolyte drinks showed their contents and some of them even gave recipes (thanks LMNT). Below are the ratios for a 16 oz glass of water.
| Element | Pedialyte Sport | LMNT | Nuun |
|---|---|---|---|
| Sodium | 650 mg | 1000 mg | 300 mg |
| Potassium | 600 mg | 200 mg | 150 mg |
| Magnesium | 55 mg | 60 mg | 25 mg |
| Phosphorous | 190 mg | - | - |
| Calcium | - | - | 13 mg |
I targeted slightly conservative values of 650mg of Sodium, 200mg of Potassium, and 55mg of Magnesium. To obtain 650mg of Sodium requires approximately 1.625g of NaCl. I needed about 0.4g of KCl to get the Potassium, and about 0.4g of Magnesium Malate to get the Magnesium. A challenge arose when using a digital kitchen scale to achieve these measurements as many scales only go down to 1g increments. I therefore 8X’ed the recipe to make a gallon.
Tasting the result was a bit strange. Luckily, I don’t think the mixing of these salts at home negatively effected me. The water was pretty salty and flavorless. It is not the sort of drink I would look forward to after a long run. Solution: 1 packet of Crystal Light. It adds 5 calories from carbohydrates which is almost nothing. It adds 20mg of sodium which is still less than the LMNT formualtion. Most importantly, it adds a bit of flavor and a sweet flavor from the asparitame.
Other options include using Sodium-bicarbonate to get the sodium levels without the chloride. Watch out for Magnesium-oxide as it is a laxative.
I should do the math on what the cost is per drink making it myself versus the store bought versions. To be continued…